Disease-Causing TIMP3 Variants and Deep Phenotyping of Two Czech Families with Sorsby Fundus Dystrophy Associated with Novel p.(Tyr152Cys) Mutation
Abstract
1. Introduction
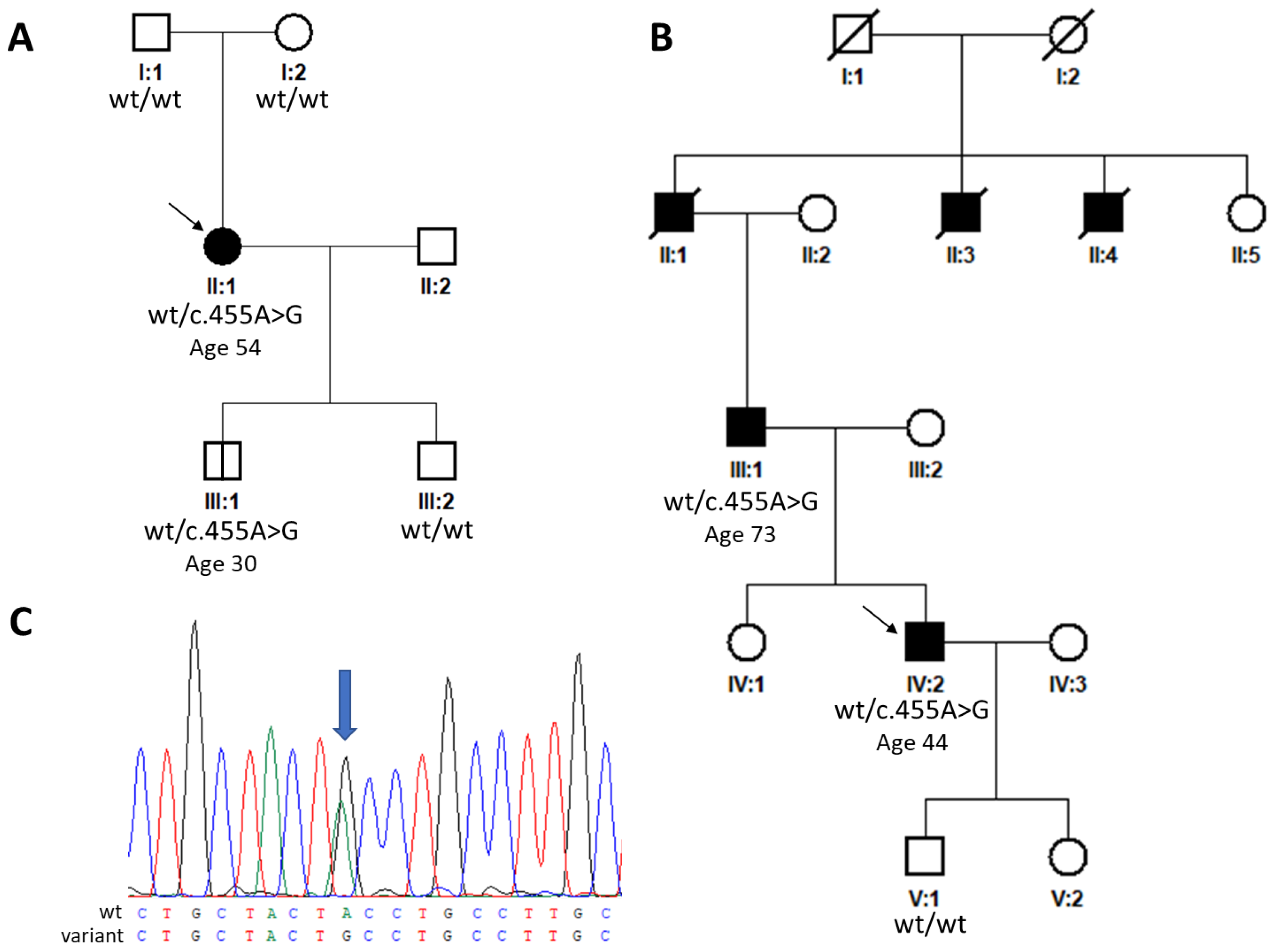
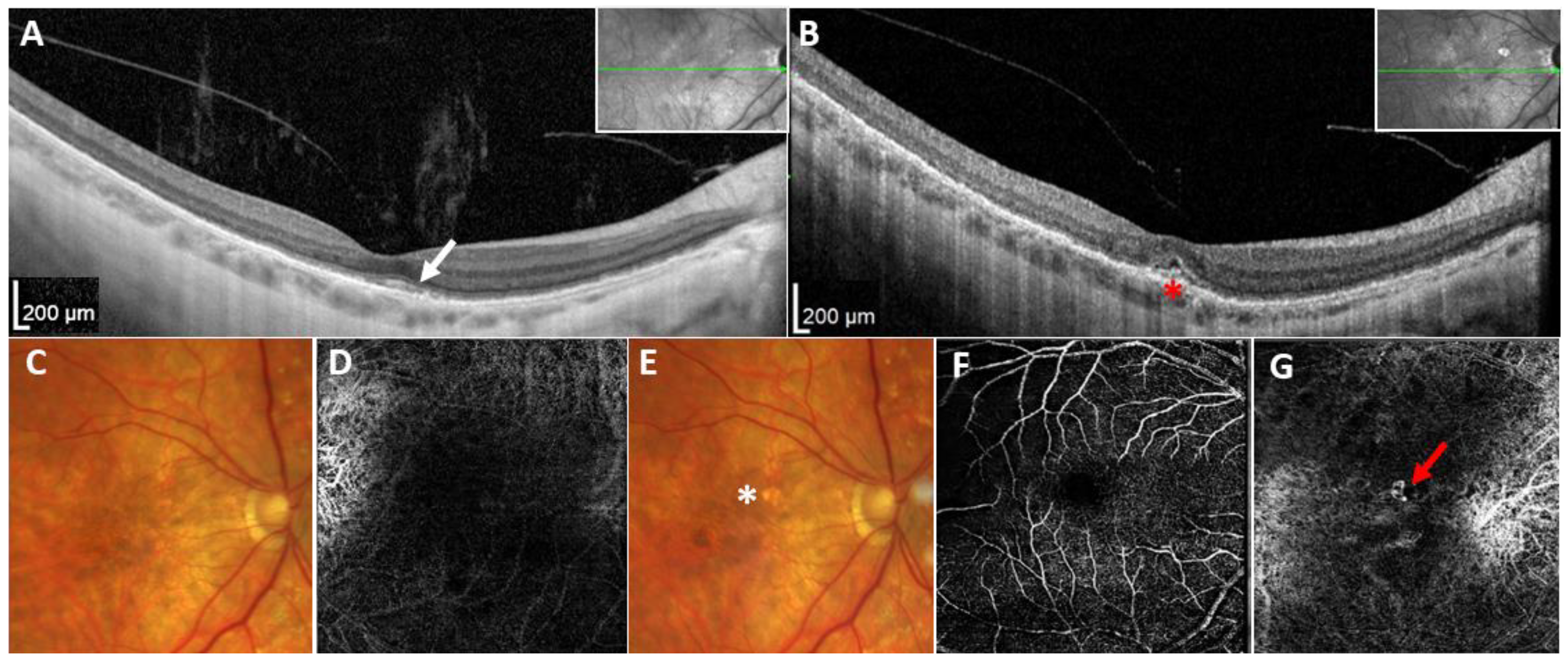
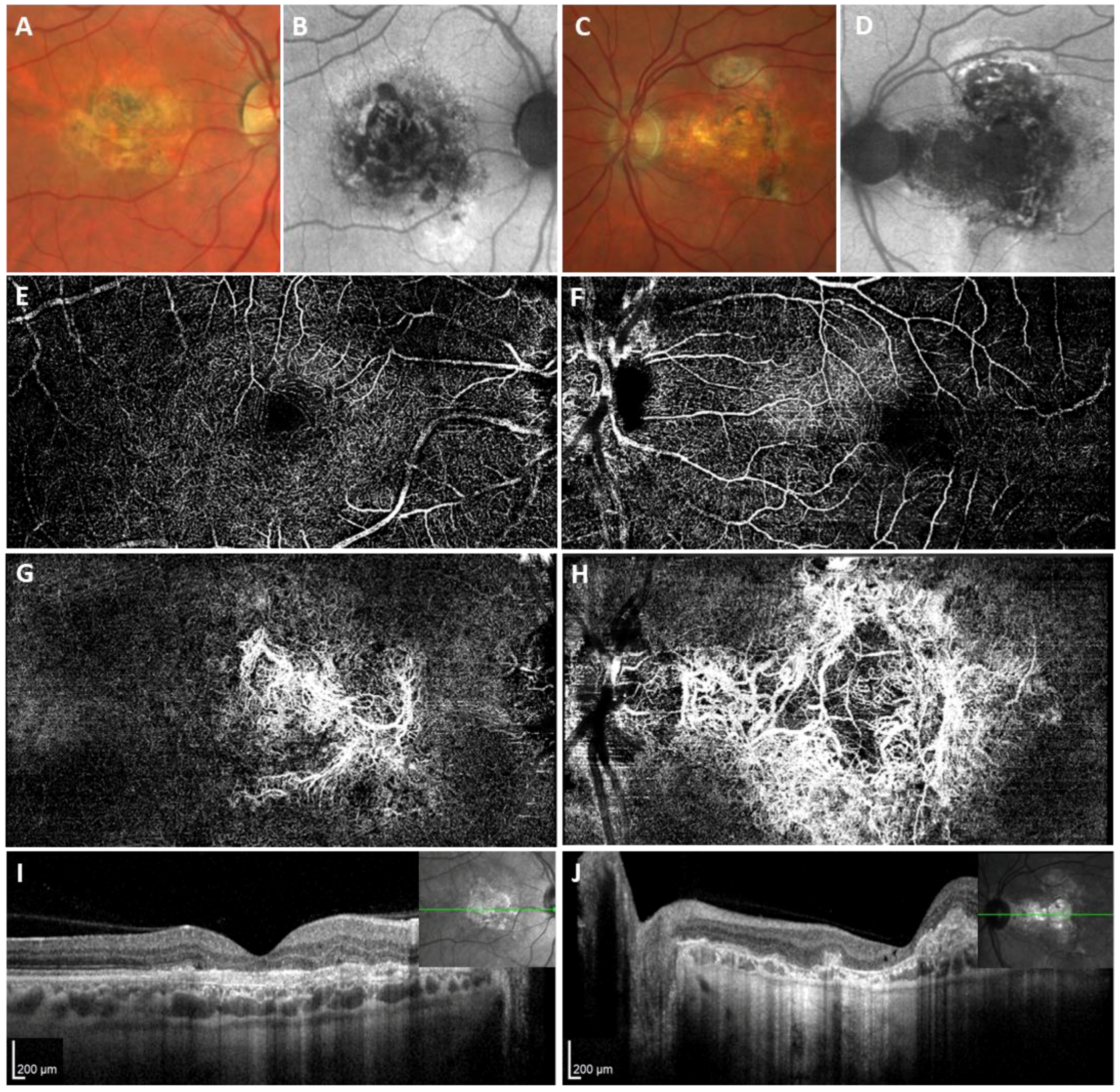
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants and Clinical Examination
4.2. Molecular Genetic Analysis
4.3. Review of TIMP3 Variants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, D.R.G.; Brown, F.E.; Cree, A.J.; Ratnayaka, J.A.; Lotery, A.J. Sorsby fundus dystrophy—A review of pathology and disease mechanisms. Exp. Eye Res. 2017, 165, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Anand-Apte, B.; Chao, J.R.; Singh, R.; Stohr, H. Sorsby fundus dystrophy: Insights from the past and looking to the future. J. Neurosci. Res. 2019, 97, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Hollyfield, J.G. TIMP-3 in Bruch’s membrane: Changes during aging and in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2367–2375. [Google Scholar]
- Gliem, M.; Muller, P.L.; Mangold, E.; Holz, F.G.; Bolz, H.J.; Stohr, H.; Weber, B.H.; Charbel Issa, P. Sorsby Fundus Dystrophy: Novel Mutations, Novel Phenotypic Characteristics, and Treatment Outcomes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2664–2676. [Google Scholar] [CrossRef]
- Tsokolas, G. Sorsby fundus dystrophy (SFD): A narrative review. Medicine 2022, 101, e30595. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A review of optical coherence tomography angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Mohla, A.; Khan, K.; Kasilian, M.; Michaelides, M. OCT angiography in the management of choroidal neovascular membrane secondary to Sorsby fundus dystrophy. BMJ Case Rep. 2016, 2016, bcr2016216453. [Google Scholar] [CrossRef]
- Spaide, R.F. Treatment of Sorsby fundus dystrophy with anti-tumor necrosis factor-alpha medication. Eye 2022, 36, 1810–1812. [Google Scholar] [CrossRef]
- Tsokolas, G.; Almuhtaseb, H.; Lotery, A. Evaluation of Pro-re-Nata (PRN) and Treat and Extend Bevacizumab treatment protocols in Sorsby Fundus Dystrophy. Eur. J. Ophthalmol. 2020, 30, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Long-Term Visual Acuity Preservation in Sorsby Fundus Dystrophy with Corticosteroid Treatment. Retin. Cases Brief Rep. 2022, 16, 44–47. [Google Scholar] [CrossRef]
- Hess, K.; Raming, K.; Gliem, M.; Charbel Issa, P.; Herrmann, P.; Holz, F.G.; Pfau, M. Choriocapillaris Flow Signal Impairment in Sorsby Fundus Dystrophy. Ophthalmologica 2022, 245, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Meunier, I.; Bocquet, B.; Labesse, G.; Zeitz, C.; Defoort-Dhellemmes, S.; Lacroux, A.; Mauget-Faysse, M.; Drumare, I.; Gamez, A.S.; Mathieu, C.; et al. A new autosomal dominant eye and lung syndrome linked to mutations in TIMP3 gene. Sci. Rep. 2016, 6, 32544. [Google Scholar] [CrossRef]
- Bloch, S.B. Implementation studies of ranibizumab for neovascular age-related macular degeneration. Acta Ophthalmol. 2013, 91, 1–22. [Google Scholar] [CrossRef]
- Guan, B.; Huryn, L.A.; Hughes, A.B.; Li, Z.; Bender, C.; Blain, D.; Turriff, A.; Cukras, C.A.; Hufnagel, R.B. Early-Onset TIMP3-Related Retinopathy Associated with Impaired Signal Peptide. JAMA Ophthalmol. 2022, 140, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Bakall, B.; Sohn, E.H.; Riley, J.; Brack, D.; Stone, E.M. Novel mutations and change of nomenclature for pathogenic variants in the TIMP3 gene causing Sorsby fundus dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3290. [Google Scholar]
- Naessens, S.; De Zaeytijd, J.; Syx, D.; Vandenbroucke, R.E.; Smeets, F.; Van Cauwenbergh, C.; Leroy, B.P.; Peelman, F.; Coppieters, F. The N-terminal p.(Ser38Cys) TIMP3 mutation underlying Sorsby fundus dystrophy is a founder mutation disrupting an intramolecular disulfide bond. Hum. Mutat. 2019, 40, 539–551. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Agarwal, A. A novel mutation at the N-terminal domain of the TIMP3 gene in Sorsby fundus dystrophy. Retina 2013, 33, 429–435. [Google Scholar] [CrossRef]
- Warwick, A.; Gibson, J.; Sood, R.; Lotery, A. A rare penetrant TIMP3 mutation confers relatively late onset choroidal neovascularisation which can mimic age-related macular degeneration. Eye 2016, 30, 488–491. [Google Scholar] [CrossRef]
- DeBenedictis, M.J.; Gindzin, Y.; Glaab, E.; Anand-Apte, B. A novel TIMP3 mutation associated with a retinitis pigmentosa-like phenotype. Ophthalmic Genet. 2020, 41, 480–484. [Google Scholar] [CrossRef]
- Tabata, Y.; Isashiki, Y.; Kamimura, K.; Nakao, K.; Ohba, N. A novel splice site mutation in the tissue inhibitor of the metalloproteinases-3 gene in Sorsby’s fundus dystrophy with unusual clinical features. Hum. Genet. 1998, 103, 179–182. [Google Scholar] [CrossRef]
- Saihan, Z.; Li, Z.; Rice, J.; Rana, N.A.; Ramsden, S.; Schlottmann, P.G.; Jenkins, S.A.; Blyth, C.; Black, G.C.; McKie, N.; et al. Clinical and biochemical effects of the E139K missense mutation in the TIMP3 gene, associated with Sorsby fundus dystrophy. Mol. Vis. 2009, 15, 1218–1230. [Google Scholar]
- Langton, K.P.; McKie, N.; Curtis, A.; Goodship, J.A.; Bond, P.M.; Barker, M.D.; Clarke, M. A novel tissue inhibitor of metalloproteinases-3 mutation reveals a common molecular phenotype in Sorsby’s fundus dystrophy. J. Biol. Chem. 2000, 275, 27027–27031. [Google Scholar] [CrossRef]
- Clarke, M.; Mitchell, K.W.; Goodship, J.; McDonnell, S.; Barker, M.D.; Griffiths, I.D.; McKie, N. Clinical features of a novel TIMP-3 mutation causing Sorsby’s fundus dystrophy: Implications for disease mechanism. Br. J. Ophthalmol. 2001, 85, 1429–1431. [Google Scholar] [CrossRef][Green Version]
- Riera, M.; Navarro, R.; Ruiz-Nogales, S.; Mendez, P.; Bures-Jelstrup, A.; Corcostegui, B.; Pomares, E. Whole exome sequencing using Ion Proton system enables reliable genetic diagnosis of inherited retinal dystrophies. Sci. Rep. 2017, 7, 42078. [Google Scholar] [CrossRef]
- Felbor, U.; Stohr, H.; Amann, T.; Schonherr, U.; Weber, B.H. A novel Ser156Cys mutation in the tissue inhibitor of metalloproteinases-3 (TIMP3) in Sorsby’s fundus dystrophy with unusual clinical features. Hum. Mol. Genet. 1995, 4, 2415–2416. [Google Scholar] [CrossRef]
- Lin, R.J.; Blumenkranz, M.S.; Binkley, J.; Wu, K.; Vollrath, D. A novel His158Arg mutation in TIMP3 causes a late-onset form of Sorsby fundus dystrophy. Am. J. Ophthalmol. 2006, 142, 839–848. [Google Scholar] [CrossRef]
- Fung, A.T.; Stohr, H.; Weber, B.H.; Holz, F.G.; Yannuzzi, L.A. Atypical sorsby fundus dystrophy with a novel tyr159cys timp-3 mutation. Retin. Cases Brief Rep. 2013, 7, 71–74. [Google Scholar] [CrossRef]
- Felbor, U.; Suvanto, E.A.; Forsius, H.R.; Eriksson, A.W.; Weber, B.H. Autosomal recessive Sorsby fundus dystrophy revisited: Molecular evidence for dominant inheritance. Am. J. Hum. Genet. 1997, 60, 57–62. [Google Scholar]
- Jacobson, S.G.; Cideciyan, A.V.; Bennett, J.; Kingsley, R.M.; Sheffield, V.C.; Stone, E.M. Novel mutation in the TIMP3 gene causes Sorsby fundus dystrophy. Arch. Ophthalmol. 2002, 120, 376–379. [Google Scholar] [CrossRef]
- Weber, B.H.; Vogt, G.; Pruett, R.C.; Stohr, H.; Felbor, U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby’s fundus dystrophy. Nat. Genet. 1994, 8, 352–356. [Google Scholar] [CrossRef]
- Barbazetto, I.A.; Hayashi, M.; Klais, C.M.; Yannuzzi, L.A.; Allikmets, R. A novel TIMP3 mutation associated with Sorsby fundus dystrophy. Arch. Ophthalmol. 2005, 123, 542–543. [Google Scholar] [CrossRef]
- Weber, B.H.; Vogt, G.; Wolz, W.; Ives, E.J.; Ewing, C.C. Sorsby’s fundus dystrophy is genetically linked to chromosome 22q13-qter. Nat. Genet. 1994, 7, 158–161. [Google Scholar] [CrossRef]
- Iyer, P.G.; Zhou, H.; Zhang, Q.; Chu, Z.; Shen, M.; Shi, Y.; Liu, J.; Trivizki, O.; Lam, B.L.; Wang, R.K.; et al. Swept-Source Optical Coherence Tomography Detection of Bruch Membrane and Choriocapillaris Abnormalities in Sorsby Macular Dystrophy. Retina 2022, 42, 1645–1654. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Rohrer, K.T.; Tindel, L.J.; Sobel, R.S.; Costanza, M.A.; Shields, W.; Zang, E. Fluorescein angiography complication survey. Ophthalmology 1986, 93, 611–617. [Google Scholar] [CrossRef]
- Wang, M.; Gao, S.; Zhang, Y.; Zhang, M. Sensitivity and specificity of optical coherence tomography angiography in the diagnosis of active choroidal neovascularization: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3529–3536. [Google Scholar] [CrossRef]
- Arris, C.E.; Bevitt, D.J.; Mohamed, J.; Li, Z.; Langton, K.P.; Barker, M.D.; Clarke, M.P.; McKie, N. Expression of mutant and wild-type TIMP3 in primary gingival fibroblasts from Sorsby’s fundus dystrophy patients. Biochim. Biophys. Acta 2003, 1638, 20–28. [Google Scholar] [CrossRef]
- Langton, K.P.; Barker, M.D.; McKie, N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby’s fundus dystrophy mutation. J. Biol. Chem. 1998, 273, 16778–16781. [Google Scholar] [CrossRef]
- Langton, K.P.; McKie, N.; Smith, B.M.; Brown, N.J.; Barker, M.D. Sorsby’s fundus dystrophy mutations impair turnover of TIMP-3 by retinal pigment epithelial cells. Hum. Mol. Genet. 2005, 14, 3579–3586. [Google Scholar] [CrossRef]
- Soboleva, G.; Geis, B.; Schrewe, H.; Weber, B.H. Sorsby fundus dystrophy mutation Timp3(S156C) affects the morphological and biochemical phenotype but not metalloproteinase homeostasis. J. Cell Physiol. 2003, 197, 149–156. [Google Scholar] [CrossRef]
- Weber, B.H.; Lin, B.; White, K.; Kohler, K.; Soboleva, G.; Herterich, S.; Seeliger, M.W.; Jaissle, G.B.; Grimm, C.; Reme, C.; et al. A mouse model for Sorsby fundus dystrophy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2732–2740. [Google Scholar]
- Rocholz, R.; Corvi, F.; Weichsel, J.; Schmidt, S.; Staurenghi, G. OCT Angiography (OCTA) in Retinal Diagnostics. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 135–160. [Google Scholar] [CrossRef]
- Ensenberger, M.G.; Thompson, J.; Hill, B.; Homick, K.; Kearney, V.; Mayntz-Press, K.A.; Mazur, P.; McGuckian, A.; Myers, J.; Raley, K.; et al. Developmental validation of the PowerPlex 16 HS System: An improved 16-locus fluorescent STR multiplex. Forensic Sci. Int. Genet. 2010, 4, 257–264. [Google Scholar] [CrossRef]
- De Bonis, P.; Laborante, A.; Pizzicoli, C.; Stallone, R.; Barbano, R.; Longo, C.; Mazzilli, E.; Zelante, L.; Bisceglia, L. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol. Vis. 2011, 17, 2482–2494. [Google Scholar]
- Gelb, B.D.; Cave, H.; Dillon, M.W.; Gripp, K.W.; Lee, J.A.; Mason-Suares, H.; Rauen, K.A.; Williams, B.; Zenker, M.; Vincent, L.M.; et al. ClinGen’s RASopathy Expert Panel consensus methods for variant interpretation. Genet. Med. 2018, 20, 1334–1345. [Google Scholar] [CrossRef]
- Mester, J.L.; Ghosh, R.; Pesaran, T.; Huether, R.; Karam, R.; Hruska, K.S.; Costa, H.A.; Lachlan, K.; Ngeow, J.; Barnholtz-Sloan, J.; et al. Gene-specific criteria for PTEN variant curation: Recommendations from the ClinGen PTEN Expert Panel. Hum. Mutat. 2018, 39, 1581–1592. [Google Scholar] [CrossRef]
- Biesecker, L.G.; Harrison, S.M.; ClinGen Sequence Variant Interpretation Working, G. The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet. Med. 2018, 20, 1687–1688. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Li, Z.; Clarke, M.P.; Barker, M.D.; McKie, N. TIMP3 mutation in Sorsby’s fundus dystrophy: Molecular insights. Expert Rev. Mol. Med. 2005, 7, 1–15. [Google Scholar] [CrossRef]
| Description | ACMG/AMP Classification | Reported Phenotype | References | |
|---|---|---|---|---|
| DNA | Protein | |||
| c.29T>A | p.(Leu10His) | Likely pathogenic | Early-onset maculopathy without CNV | [16] |
| c.34G>C | p.(Gly12Arg) | Likely pathogenic | Early-onset maculopathy without CNV | [16] |
| c.70T>C | p.(Cys24Arg) | Likely pathogenic | SFD (no clinical description) | [17] |
| c.113C>G | p.(Ser38Cys) | Pathogenic | SFD | [17,18,19,20] |
| c.410A>G | p.(Tyr137Cys) | Likely Pathogenic | Retinitis pigmentosa-like, drusen | [21] |
| c.439-2dup | p.? | Likely Pathogenic | SFD | [22] |
| c.452A>G | p.(Tyr151Cys) | Likely Pathogenic | SFD (no clinical description) | [17] |
| c.455A>G | p.(Tyr152Cys) | Pathogenic | SFD | Current study |
| c.484G>A | p.(Glu162Lys) | Likely pathogenic | SFD | [23] |
| c.484G>T | p.(Glu162*) | Likely pathogenic | SFD | [24,25] |
| c.499G>A | p.(Asp167Asn) | VUS | MD vs. SFD (no clinical description) | [26] |
| c.521A>G | p.(Tyr174Cys) | Pathogenic | SFD | [6] |
| c.530A>G | p.(Tyr177Cys) | Pathogenic | SFD | [6] |
| c.536C>G | p.(Ser179Cys) | Pathogenic | SFD | [24,27] |
| c.542A>G | p.(His181Arg) | Pathogenic | SFD | [28] |
| c.545A>G | p.(Tyr182Cys) | Pathogenic | SFD | [6,29] |
| c.565G>T | p.(Gly189Cys) | Pathogenic | SFD | [24,30] |
| c.568G>T | p.(Gly190Cys) | Pathogenic | SFD | [31] |
| c.572A>G | p.(Tyr191Cys) | Likely pathogenic | SFD | [32] |
| c.577A>T | p.(Ser193Cys) | Likely pathogenic | Retinitis pigmentosa-like, drusen | [33] |
| c.584A>G | p.(Tyr195Cys) | Pathogenic | SFD | [31] |
| c.594G>T | p.(Trp198Cys) | Likely pathogenic | SFD (no clinical description) | [17] |
| c.610A>T | p.(Ser204Cys) | Pathogenic | SFD | [32,34] |
| Pathogenic Criteria | ACMG/AMP Criteria | Strong | Moderate | Supporting | |
|---|---|---|---|---|---|
| Population data | PM2 | Absent in population databases | gnomAD non-founder subpopulations frequency 0.0% | gnomAD non-founder subpopulations frequency <0.0001% | |
| PS4 | The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls | ≥5 observations | 3–4 observations | 2nd independent occurrence | |
| Computational and predictive data | PP3 | Multiple lines of computational evidence support a deleterious effect on the gene or gene product | REVEL score > 0.932 | REVEL score (0.773–0.932) | REVEL score (0.644–0.772) or SpliceAI score > 0.5 for splicing variants |
| Phenotype | PP4 | Patient’s phenotype or family history is highly specific for a disease with a single genetic aetiology | Clinical diagnosis of SFD with consistent phenotype description | Clinical diagnosis of SFD without phenotype description | Clinical diagnosis of macular dystrophy or retinitis pigmentosa without CNV and/or drusen with phenotype description |
| Functional data | PS3 | Well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product | Studies support functional impact | ||
| PM1 | Located in a mutational hot spot and/or functional domain without benign variation | Variant leads to the introduction or loss of a cysteine | |||
| Segregation data | PP1 | Co-segregation with disease in multiple affected family members in a gene definitively known to cause the disease | Co-segregation with disease ≥7 meiosis | Co-segregation with the disease in 5–6 meiosis | Co-segregation with the disease in 3–4 meiosis |
| De novo data | PS2 | De novo in a patient with disease and no family history | De novo in a patient with phenotype consistency; no family history and both maternity and paternity are confirmed | ||
| PM6 | De novo in a patient with phenotype consistency; no family history and both maternity and paternity are assumed | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergaro, A.; Pankievic, M.; Jedlickova, J.; Dudakova, L.; Vajter, M.; Michaelides, M.; Meliska, M.; Nemec, P.; Babincova, D.; Kousal, B.; et al. Disease-Causing TIMP3 Variants and Deep Phenotyping of Two Czech Families with Sorsby Fundus Dystrophy Associated with Novel p.(Tyr152Cys) Mutation. Int. J. Mol. Sci. 2024, 25, 3744. https://doi.org/10.3390/ijms25073744
Vergaro A, Pankievic M, Jedlickova J, Dudakova L, Vajter M, Michaelides M, Meliska M, Nemec P, Babincova D, Kousal B, et al. Disease-Causing TIMP3 Variants and Deep Phenotyping of Two Czech Families with Sorsby Fundus Dystrophy Associated with Novel p.(Tyr152Cys) Mutation. International Journal of Molecular Sciences. 2024; 25(7):3744. https://doi.org/10.3390/ijms25073744
Chicago/Turabian StyleVergaro, Andrea, Monika Pankievic, Jana Jedlickova, Lubica Dudakova, Marie Vajter, Michel Michaelides, Martin Meliska, Pavel Nemec, Daniela Babincova, Bohdan Kousal, and et al. 2024. "Disease-Causing TIMP3 Variants and Deep Phenotyping of Two Czech Families with Sorsby Fundus Dystrophy Associated with Novel p.(Tyr152Cys) Mutation" International Journal of Molecular Sciences 25, no. 7: 3744. https://doi.org/10.3390/ijms25073744
APA StyleVergaro, A., Pankievic, M., Jedlickova, J., Dudakova, L., Vajter, M., Michaelides, M., Meliska, M., Nemec, P., Babincova, D., Kousal, B., & Liskova, P. (2024). Disease-Causing TIMP3 Variants and Deep Phenotyping of Two Czech Families with Sorsby Fundus Dystrophy Associated with Novel p.(Tyr152Cys) Mutation. International Journal of Molecular Sciences, 25(7), 3744. https://doi.org/10.3390/ijms25073744






